Fuel cell is a kind of power generation device, but it is not like a normal non-rechargeable battery and discarded when used up, nor is it like a rechargeable battery. To maintain its electricity, the fuel required is “hydrogen”, and this is why it is classified as a new energy source.
Principle of fuel cells
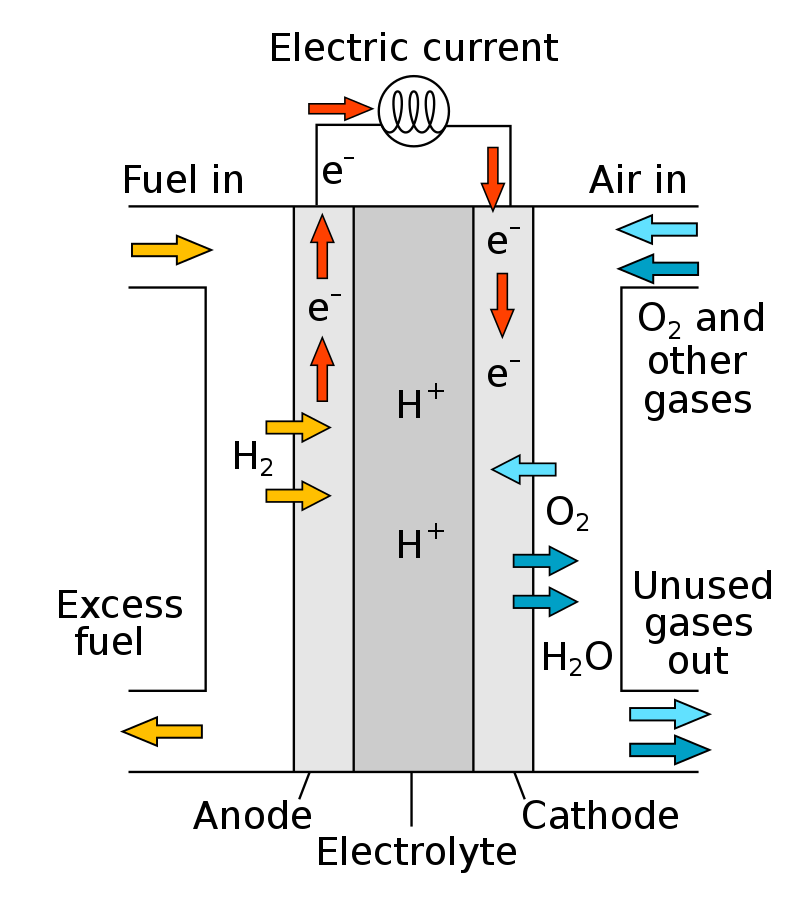
The battery contains two electrodes, an anode and a cathode, which are respectively filled with electrolyte, and the two electrodes are composed of a permeable membrane. Hydrogen enters the anode of the fuel cell, and oxygen (or air) enters the fuel cell from the cathode. Through the action of the catalyst, the hydrogen molecule at the anode is decomposed into two protons and two electrons. The protons are attracted by oxygen to the other side of the film, and the electrons form a current through the external circuit and reach the cathode. Under the action of the cathode catalyst, protons, oxygen and electrons react to form water molecules, so water can be said to be the only emission from fuel cells.
The “hydrogen” fuel used in fuel cells can come from hydrogen produced by the electrolysis of water; And any hydrocarbons, such as natural gas, methanol, ethanol (alcohol), biogas, and so on.
Because the fuel cell generates electricity and water through the chemical reaction of hydrogen and oxygen. It is not only completely pollution-free, but also avoids the time-consuming problem of traditional battery charging. It is currently the most promising new energy method. If it can be popularized and applied in vehicles and other high-polluting power generation tools, it will significantly reduce air pollution and the greenhouse effect.
Principle of Hydrogen fuel cell vehicle
The working principle of the hydrogen fuel cell vehicle is: send hydrogen to the anode plate (negative electrode); Through the action of the catalyst (platinum), an electron in the hydrogen atom is separated. and the hydrogen ion (proton) that has lost the electron passes through the proton exchange membrane reaches the cathode plate. Electrons cannot pass through the proton exchange membrane. This electron can only reach the cathode plate of the fuel cell through the external circuit. Thereby generating current in the external circuit. After the protons reach the cathode plate, they recombine with oxygen atoms and hydrogen ions to form water.
Since the oxygen supplied to the cathode plate can be obtained from the air. As long as the anode plate is continuously supplied with hydrogen, and the cathode plate is supplied with air. The water (vapor) is taken away in time, electric energy can be continuously supplied.
The electricity generated by the fuel cell is supplied to the electric motor through inverters, controllers and other devices. Then the wheels are driven to rotate through the transmission system, drive axle, etc. So that the vehicle can drive on the road.
Compared with traditional vehicles, the energy conversion efficiency of fuel cell vehicles is as high as 60 to 80%. Which is 2 to 3 times that of internal combustion engines.
The fuel of the fuel cell is hydrogen and oxygen, and the product is clean water. It does not produce carbon monoxide and carbon dioxide, nor does it emit sulfur and particulates. Therefore, hydrogen fuel cell vehicles are truly zero-emission and zero-pollution vehicles, and hydrogen fuel is the perfect vehicle energy source!