titanium paper are mainly used as flow field or diffusion material in electrolytic cells, especially on the oxygen (anode) side. Since carbon is immediately oxidized to carbon dioxide (if the medium is acidic) or carbonate ions (if the medium is alkaline) during the electrolysis reaction, electrolytic cell hardeners are not able to use carbon-based Gas Diffusion Layers (GDLs) on oxygen evolution electrodes, which makes titanium paper an ideal candidate for use as a diffusion medium, as well as to provide an electrical contact between the anodic catalyst layer and the anodic bipolar plate or the anodic current collecting element. Our titanium paper are made from small diameter titanium fibers that have been compressed into a flat shape and annealed (i.e., sintered) to ensure a smooth, flat surface for use in electrolytic cells. While most industrial applications utilize electrolyzers in an anode-fed mode, this product is also an ideal diffusion medium for researchers conducting studies on cathode-fed electrolyzers.
While most industrial applications run their electrolyzers in an anode-fed mode and this product is an ideal diffusion medium for oxygen evolution electrodes, it can also be used as an ideal diffusion medium for researchers conducting cathode-fed electrolyzer studies.
For unitized fuel cells or electrolysers or regenerative electrochemical device systems, titanium paper can be used as diffusion media at the anode or cathode. Regardless of the nature of the reactants, electrochemical oxygen concentrators, electrochemical fire extinguishers, and certain types of batteries (e.g., redox flow batteries) can also use this product as a diffusion medium.
When should I use titanium paper?
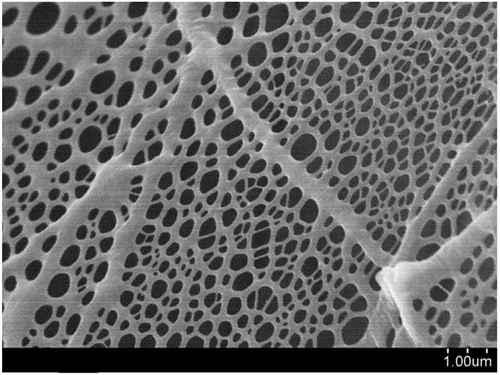
Untreated titanium paper are not consumed as much as carbon gas diffusion layers (GDL), however, the presence of oxygen can have an effect if the electrolyzer is operated at high pressures (1 bar to 3 bar). At high oxygen pressures, the untreated titanium surface will rapidly form an electrically insulating oxide layer (TiO2) on the surface of the small diameter fibers, which will ultimately affect the efficiency of the entire system. This oxide coating will act as an electrical insulator and therefore increase the interfacial resistance in the cell, thus reducing electrochemical performance.
A gold or platinum coating prevents oxidation of the titanium fibers in the felt. Platinization of the titanium surface produces a coating that is electrically conductive and chemically stable under conventional electrolytic operating conditions and extends the life of the titanium block, making it ideal for applications requiring high performance and long life. Preventing the formation of TiO2 will greatly stabilize the electrochemical performance of the electrolyzer or intended electrochemical device.