Precious metal catalysts
Noble metal platinum-based catalysts
Noble metal platinum-based catalysts (PGMs) are the best performing HER catalysts available. It has low overpotential, high exchange current density and remarkable reaction stability. It can effectively reduce the hydrolysis energy barrier and increase the reaction rate. The disadvantage is that resources are scarce and expensive, which limits its large-scale application to a certain extent.
At present, the research on precious metal catalysts mainly focused on the regulation of structure and composition. By changing the structure of the noble metal catalyst, the electrochemical performance can be substantially improved. Or combine it with other non-precious metal materials to reduce the loading and cost while maintaining its catalytic activity.
Design of single-atom catalysts (SACs) by dispersing the metal active center on the catalyst carrier at the atomic level. It is an effective strategy to enhance HER activity. Conventional PGMs have low atomic utilization and low catalytic efficiency because the active sites are only derived from surface-exposed Pt.
In contrast, the single-atom form is able to increase the amount of active material on a limited surface. And fully expose the highly active corner and edge sites of the catalyst particles. This can effectively improve the catalytic efficiency.
Pt/OLC catalyst
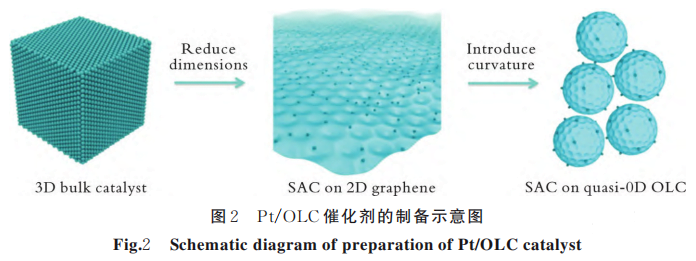
D.B. Liu et al. prepared Pt/OLC catalysts using onion-like carbon nanospheres (OLC) anchored with Pt atomic particles. And the schematic diagram of their preparation is shown in Figure 2.
The experimental results show that the material has a low overpotential of 38 mV at a current density of 10 mA/cm2. And its high curvature structure effectively enhances the local electric field at the Pt site, thus promoting the reaction kinetics.
L. Zhang et al. [used nitrogen-doped carbon nanotubes (CNT) as a carrier for Pt deposition and found that the nitrogen-rich carrier facilitated the deposition of Pt single atoms and exhibited better HER activity and stability than commercial Pt/C catalysts. In addition to C carriers, many studies have successfully synthesized SACs using MoS2, FeOx, and TiN as carriers, effectively reducing the Pt loading.
Precious metal ruthenium (Ru) catalysts
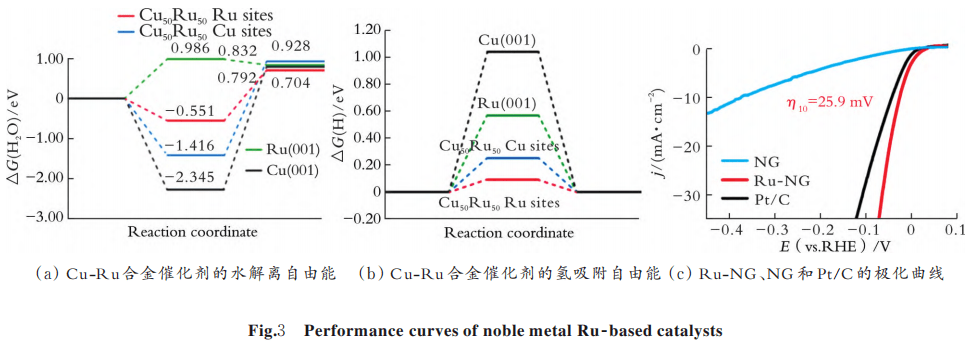
The noble metal ruthenium (Ru) also has good electrochemical properties and is less expensive than Pt, but it is less stable and prone to agglomeration. It can solve by alloying, elemental doping and matrix anchoring. Q.L. Wu et al. prepared a three-dimensional nanoporous Cu⁃Ru alloy by the alloying method, and calculated the HER activation energy by density flooding theory.
In Fig. 3, the alloy effectively reduces the water dissociation energy barrier and optimizes. the hydrogen adsorption ⁃desorption energy compared to pure Cu or Ru. The HER activity of the catalyst in alkaline/neutral media was significantly improved. Y. Li et al. uniformly deposited Ru nanoclusters in nitrogen-doped graphene (NG) by a two-step pyrolysis method with a low overpotential of 25.9 mV at a current density of 10 mA/cm2 (see Fig. 3(c)), which enables efficient basic HER.
F. Li et al. utilized the reported carboxylated graphene nanosheets (CGnP) to anchor Ru3+. They uniformly loaded with Ru nanoparticles after annealing reduction and exhibited HER activity comparable to that of commercial Pt/C catalysts in acid/base media.
Transition metal catalysts
Transition metal(TM) has good catalytic activity and is an ideal material to replace precious metal catalysts. Because its low price and abundant reserves. For example, transition metals and their alloys, oxides, sulfides, nitrides, phosphides, etc., all have excellent HER activity and stability. The performance of some of these catalysts is comparable to that of precious metal catalysts and has broad application prospects.