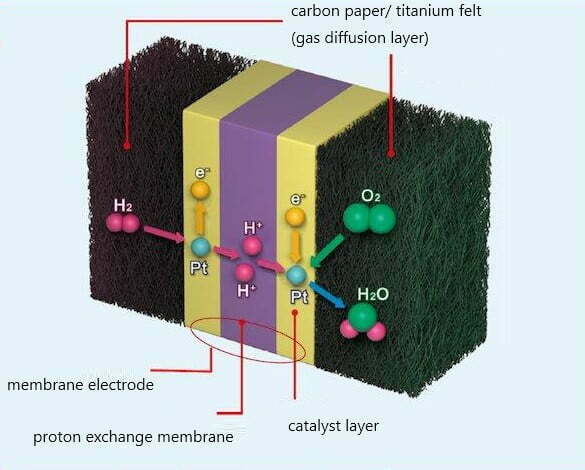
Membrane electrode assembly, also known as membrane electrode, is the key core component of fuel cell power generation, which is composed of gas diffusion layer, catalyst layer and proton exchange membrane.
Principle
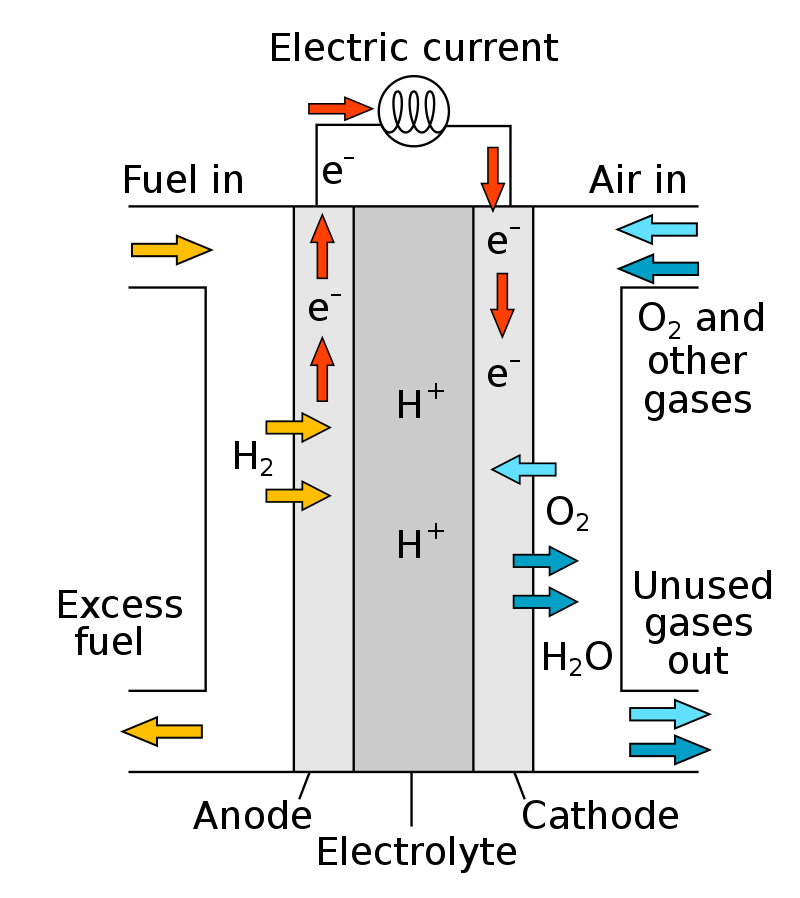
Hydrogen reaches the anode through the gas flow field on the anode plate, reaches the anode catalyst layer through the diffusion layer on the electrode, and is adsorbed on the anode catalyst layer. The hydrogen gas is decomposed into 2 hydrogen ions, namely proton H+, under the catalytic action of the catalyst platinum. Release 2 electrons. This process is called hydrogen anodic oxidation process.
Anode: 2H2=4H++4e–
At the other end of the battery, oxygen or air reaches the cathode through the gas flow field on the cathode plate, reaches the cathode catalyst layer through the diffusion layer on the electrode, and is adsorbed on the cathode catalyst layer. At the same time, hydrogen ions pass through the electrolyte to the cathode, and electrons pass through The external circuit also reaches the cathode. Under the action of the cathode catalyst, oxygen reacts with hydrogen ions and electrons to produce water. This process is called the cathode reduction process of oxygen.
Cathode: O2+4H++4e– = 2H2O
Total: 2H2+O2=2H2O
At the same time, the electrons form a current under the connection of the external circuit, and can output electrical energy to the load through proper connection, and the generated water is discharged with the reaction tail gas through the electrode.
Features
- Firstly, it can minimize the transmission resistance of the gas, so that the reaction gas can smoothly reach the catalytic layer from the diffusion layer to cause an electrochemical reaction.
- Secondly, form a good ion channel to reduce the resistance of ion transmission.
- Thirdly, form a good electronic channel.
- Fourth, the gas diffusion electrode should ensure good mechanical strength and thermal conductivity.
- Fifth, The membrane has high proton conductivity.
Summary
Proton exchange membrane is sandwiched between two electrodes, and the catalyst is embedded between them. Electrode is insulated from the proton exchange membrane. The two electrodes are divided into anode and cathode.
The proton exchange membrane is a proton permeable membrane, which is insulating. Protons are transported from the anode to the cathode through this insulating film, and electrons are transported from the conductive path to the cathode.
The electrode is hot pressed on the PEM. Commonly used electrode materials include carbon fiber, carbon fiber paper, and a new material —titanium fiber felt. The carbon fiber produced by E-TEK is called ELAT, which can maximize the transport of gas to the PEM while removing water from the PEM. The precious metal catalyst is embedded in elat, which can be used as an electrode. Platinum is one of the most commonly used catalysts, but other platinum group metals are also frequently used. Ruthenium and platinum are often used together.
Besides, if CO is used as the product of an electrochemical reaction. It will destroy the proton exchange membrane and affect the efficiency of the fuel cell.
Due to high cost of rare earth metals, the development of low-cost catalyst materials has become a hot research topic. It is precisely because of these high cost factors that have become an obstacle to the promotion of fuel cells.
In practical applications, fuel cell stack can meet the needs of different sizes of power output according to the needs of the design.