Efficient catalysts appear to be important for green hydrogen preparation, chemical industry, fertilizer production and other economic sectors. In addition to the use of transition metals, various other metallic or non-metallic elements have now become the focus of research.
Green hydrogen is an important component in a climate-neutral energy system. It is produced by electrolytically splitting water with wind or solar power and stores this energy in chemical form. But currently, the production of green hydrogen is not yet economical or efficient enough. The key to solving this problem is through the development of innovative electrocatalysts. It should not only work with high efficiencies but should also be available and inexpensive.
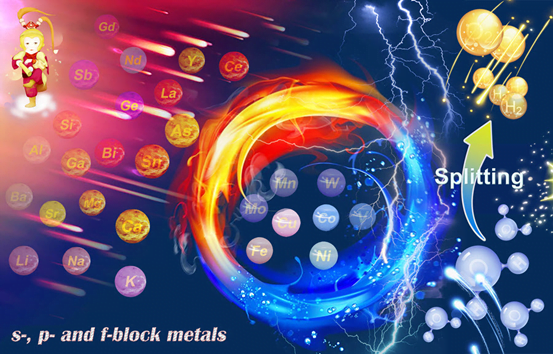
In addition to transition metals, which are already well studied for their catalytic properties, a wider choice of elements has now moved into the focus such as alkali metals, alkaline earth metals, rare earth metals, lean metals and metalloids. Some of these when combined with transition metal electrocatalysts can significantly improve performance. And contribute to the development of next-generation high-performance electrocatalysts.
However, many of the processes that take place during electrocatalysis. When oxygen or hydrogen how to form can’t understand in detail. In a review article, an international team of experts guides us through this exciting research field and draws a perspective. Sketching the next steps catalyst research could take. “This contribution summarises the current state of knowledge on such unconventional s-, p-, and f-block metal-based materials and makes it comprehensible to a wider community of scientists,”