Electrolytic water hydrogen production technology is still not mature enough compared to traditional fossil energy hydrogen production technology. Now the biggest disadvantage of the whole set of mechanism of electrolytic water hydrogen production technology is the cost. However, electrolytic water hydrogen production has the advantages of simple process, no pollution and high purity of output hydrogen. This can be well combined with renewable energy to achieve a significant reduction in the cost of hydrogen production. Currently, electrolysis of fresh water for hydrogen production is widely used. However, freshwater reserves account for only 2.53% of all water resources on Earth. And a great part of freshwater is stored in icebergs and glaciers, which is difficult to be used by humans. According to the survey, only 0.3% of the total freshwater reserves can be used by human beings.
Vigorously develop the electrolysis of seawater to produce hydrogen industry. And then combine with the relatively mature offshore wind power system along our coast. This can realize a relatively perfect hydrogen energy production industry with large scale and no pollution. The main electrolytic water hydrogen production technologies are alkaline water electrolysis hydrogen production technology. And proton exchange membrane water electrolysis hydrogen production technology and solid oxide water electrolysis hydrogen production technology. At present, the United States, Japan, Korea and Europe all regard electrolytic water hydrogen production technology as the mainstream development direction of future energy technology. The main focus is to scale up the alkaline water electrolysis hydrogen generation technology. And industrialize the proton exchange membrane water electrolysis hydrogen generation technology. We will focus on the three key performance indicators of electrolysis efficiency, durability and equipment cost to promote the overall technology development.
(1) Principle of hydrogen production by electrolysis of water.
The basic principle of electrolysis of water is to use electrical energy as a source of energy to drive the electrochemical reaction of water molecules on an electrode to produce hydrogen and oxygen.
Electrolytic water process reaction equation:2H2O→2H2+O2
Cathode (hydrogen precipitation) under alkaline conditions (industrial conditions):2H2O+2e-→H2+2OH-
Anode (oxygen precipitation):2OH-→H2O+2e-+0.5O2
(2) Opportunities for hydrogen production from electrolysis of seawater
With 32,000 km of coastline, China is extremely rich in seawater reserves. This brings a rich raw material base for the hydrogen energy manufacturing industry. Seawater hydrogen production does not depend on freshwater resources and is suitable for electrolysis of water for hydrogen production in coastal and other areas. The ionic conductivity of seawater is much higher than that of freshwater due to various ions in seawater. There is no need to add acidic or alkaline substances to increase the conductivity of the solution during electrolysis, which further reduces the cost.
Secondly, there are abundant wind energy resources in the sea, and wind energy is a high-quality clean energy. Wind power generation is the most mature, large-scale development and commercial development prospect of new energy generation technology. Wind power coupled with electrolytic water hydrogen production technology expected to be the key to break the game of offshore wind power development in deep and distant sea.
Projects such as PosHYdon project in the Netherlands and H2Ouest project in France represent the booming development of hydrogen production from wind power abroad. In China, the scale of offshore wind power hydrogen production projects is also ready to develop – Zhejiang, Fujian, Shanghai and other places have proposed to explore the development of offshore wind power hydrogen production projects. Hydrogen energy towards deep and distant sea has a broad application prospect and is an inevitable trend.
The development of efficient technical means of hydrogen production from seawater is an important link to promote hydrogen energy to the deep and distant sea. The efficient utilization of hydrogen is also an important part of the project to improve
hydrogen energy to the deep and distant sea. Compared with the transmission to land through pipeline, the distributed hydrogen refueling station for direct use by transport vessels can further save cost. If the ocean-going vessels can use hydrogen as the driving fuel, they can replenish the energy through the mobile hydrogen refueling station at sea and increase their own capacity to create more revenue.
(3) Challenges of electrolysis of seawater for hydrogen production
While electrolysis of seawater for hydrogen production has a very promising future, it also faces great challenges. Geographically, the challenge of hydrogen production from electrolytic water is that the industry can only develope in coastal areas. It is difficult for inland cities to participate in the seawater hydrogen production project. As the most promising energy technology of the future, hydrogen from electrolytic water has to apply in all coastal areas of the world. However, low-temperature water electrolyzer is now a more mature technology for hydrogen production, which requires high purity water raw material. If electrolytic water to hydrogen technology is more widely deployed to hot and arid coastal areas around the world. These areas have limited fresh water resources, but have large amounts of ocean seawater. Therefore, seawater resources can be a bottleneck for electrolytic water to hydrogen technology.
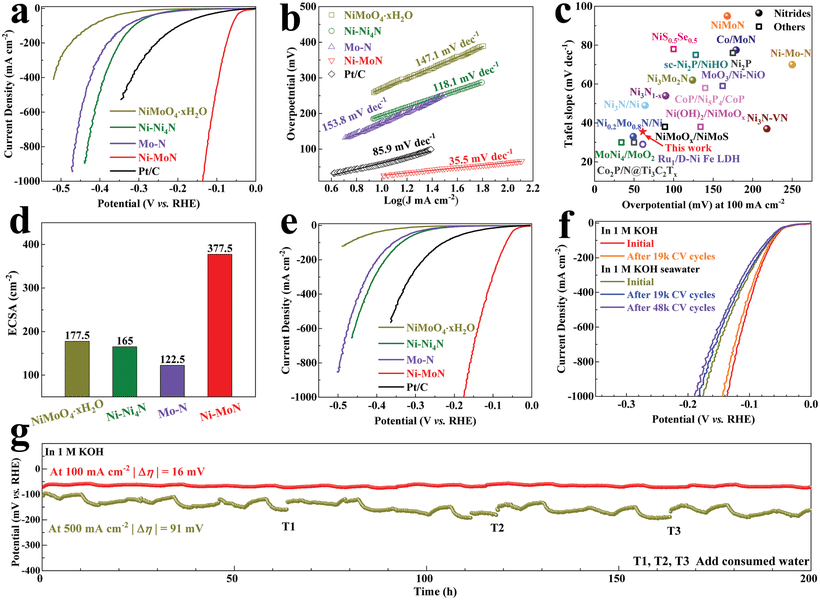
Electrolysis of seawater requires corresponding catalysts, and the development of highly stable, low-cost and active catalysts is the key to research. In freshwater electrolysis, platinum-based catalysts have shown excellent properties. However, the performance in seawater is not satisfactory, and the cost of platinum-based catalysts is too high to use for large-scale industrial electrolysis of water. Researchers are currently working on non-precious metal catalysts. For example, transition metal sulfides, selenides. And carbides, such directions expected to investigate catalytic materials with larger exchange current densities and smaller Tafel slopes.
A researcher has adopted the membrane based alkaline seawater electrolysis technique. While simulating alkaline conditions with KOH and adding NaCl to synthesize 0.5 M seawater for electrolysis. Instead, they found that after a long period of electrolysis, chloride ions hindered the membrane’s ability to move hydroxide. It causes the current density in the electrolyte to decrease dramatically. Thankfully, however, the researchers did not find any negative effects of NaCl on the electrodes and catalysts in their tests.