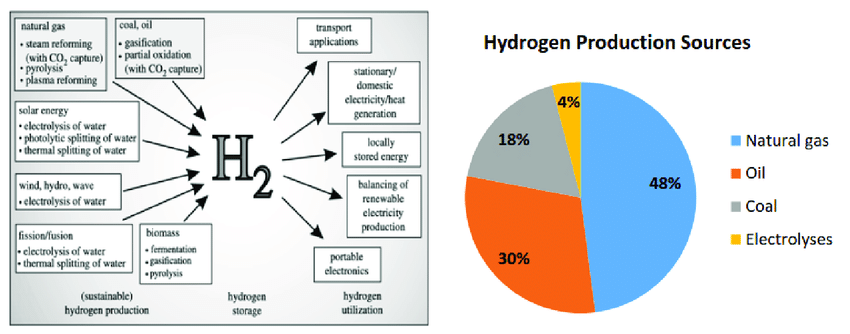
At present, there are many methods to produce hydrogen energy. It can be roughly divided into the following categories according to the different ways and principles of raw material conversion: solar thermal chemical hydrogen production, fossil energy hydrogen production, biomass hydrogen production and electrolysis of water hydrogen production, etc.
(1) Solar thermal chemical hydrogen production
Solar thermochemical reaction cycle for hydrogen production, also known as indirect pyrolysis of water for hydrogen production. Compared with direct pyrolysis for hydrogen production. The indirect method overcomes the problem of high temperature (its reaction temperature is only 900-1200K). The dependence on equipment and materials greatly reduced and safety is greatly improved. If the solar energy directly to the thermal decomposition of water, H2 and O2 two gases are more difficult to separate.
At the same time the reaction is reversible and hydrogen and oxygen may recombine at high temperatures to form water and may even explode. And the H2 and O2 separated by themselves during the reaction of indirect pyrolysis of water to hydrogen. It is a good solution to this technical problem.
Although the method has less greenhouse gas emissions, it is currently expensive to build. The technology is not mature enough and needs further improvement to meet market requirements on a large scale.
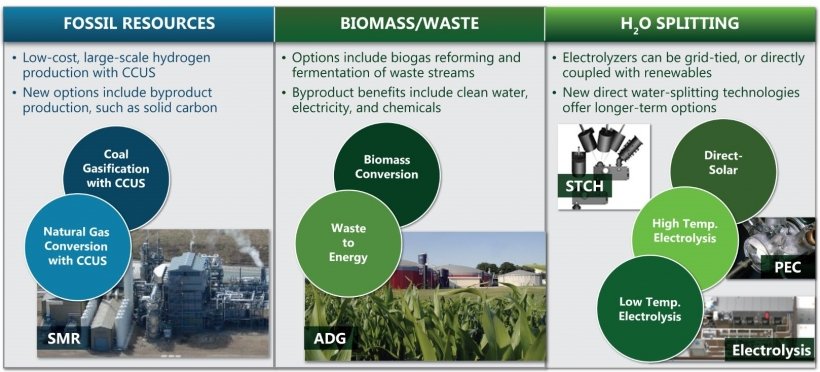
(2) Fossil energy to hydrogen
Today’s industrial-scale hydrogen production is still mainly from fossil fuels, with roughly 92% of the world’s hydrogen produced from coal and natural gas. As the main hydrogen production method, fossil fuel hydrogen production has the advantages of mature technology. The low raw material cost and large scale of plant. Among them, coal to hydrogen and natural gas to hydrogen are the most important ways to produce hydrogen.
Coal gasification to hydrogen technology is used in industrial large-scale hydrogen production processes. The specific process involves gasification of coal at high temperature to produce water gas, conversion of CO and water vapor to H2 and CO2. Removal of acid gases (such as CO2 and SO2), and purification of hydrogen. As a result, hydrogen of different purity can obtain. Coal-to-hydrogen technology now used on a large scale in industrial production and favored because of its low cost and high technological maturity. However, the low efficiency of hydrogen production and the high greenhouse gas emissions are the main reasons that limit the further diffusion of the coal-to-hydrogen process.
There are two main methods to prepare hydrogen from methane, one is to prepare water gas first and then get hydrogen. Or directly decomposing methane to obtain hydrogen. Both methods require the activation of methane molecules first. However, because methane molecules are very inert, successful activation requires more demanding conditions. Even though methane can produce syngas at temperatures below 700K. However, high levels of hydrogen yield can only achieve at temperatures above 1100 K. Compared to the coal-to-hydrogen process, methane to hydrogen has lower greenhouse gas emissions and is a relatively ideal method for industrial hydrogen production. However, the problems of high energy consumption, high production cost and high investment in equipment need to solved.
(3) Biomass to hydrogen
The phenomenon of biological photosynthetic hydrogen production discovered by Gest as early as 1949. And Lewis proposed the idea of biological hydrogen production in 1966. According to the different types of hydrogen producing microorganisms utilized in biohydrogen production technology. Generally, it can divide into two categories: anaerobic fermentation hydrogen production and photosynthetic biogenic hydrogen production. Biomass hydrogen production has the advantages of low energy consumption, low greenhouse gas emission and easy access to raw materials. And can theoretically have a large hydrogen production capacity. However, the composition of raw materials is complicated, the primary products have many impurities, and the purification process is difficult and covers a large area, which is not suitable for large-scale production.
There are three basic pathways for biological hydrogen production through anaerobic fermentation. They mixed acid fermentation, butyric acid type fermentation, and NADH pathway. The main mechanism of hydrogen production by NADH pathway is that glucose is fermented to produce pyruvate under anaerobic conditions. At the same time, a large amount of NADH and H produced. And when the NADH and H in the microorganism accumulate to a certain level. The NADH will release molecular hydrogen through the action of hydrogenase in the microorganism. And both pathways, butyric acid type fermentation and mixed acid fermentation, occur in pyruvate decarboxylation. They are a special regulatory mechanism adopted by the microorganisms themselves to resolve the “excess” electrons produced during these two fermentations.
Photosynthetic bacterial hydrogen production accomplished by photocracking organic acids, rather than simply photolyzing water. The photosynthetic hydrogen production pathway catalyzed by nitrogen fixing enzymes or hydrogenases. Coupling photosynthetic phosphorylation with the metabolism of reducing substances. A process that uses the absorbed light energy with the reducing power generated by metabolism to produce hydrogen.